On 22 July 2014, the Head of the Presidential Secretariat (HPS) announced that only one company met the prequalification requirements for the supply of drugs and medical supplies to the Ministry of Health and the Georgetown Hospital. The HPS disclosed that Cabinet offered its no-objection to the recommendation of the National Procurement and Tender Administration Board (NPTAB). The company will therefore be the only local supplier for the next three years, joining five specialized agencies from overseas that were prequalified previously.
Some eight months earlier, the Ministry of Health had advertised for manufacturers and suppliers of pharmaceutical products to apply for prequalification based on a revised set of criteria. In the previous prequalification exercise covering the period 2011 to 2014, suppliers were required to fill out a four-part questionnaire dealing with information relating their business, manufacturing capability, quality control and product details. There was, however, no indication in the package of prequalification documents as to the agreed evaluation criteria to be used to enable prospective suppliers to ascertain how they would be evaluated. The only exception was that a supplier must be able to supply 75 per cent of the required items.
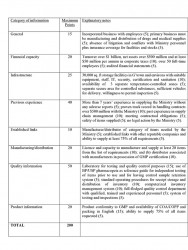
Revised Evaluation Criteria
Section 6 (5) of the Procurement Act states that in reaching a decision regarding the qualifications of each supplier or contractor, the procuring entity is required to apply only the criteria set forth in the prequalification documents. To address the deficiency in the previous pre-qualification exercise, the Ministry developed a points system to be used in the evaluation exercise, as summarised below:
Manufacturers were evaluated on a score of 190 while for distributors the score is 180. At least 80 per cent was required for prequalification in addition to meeting the criteria dealing with financial and infrastructure capacity as well as the ability to supply 75 per cent of GMA certified items. Pharmaceutical manufacturers in Guyana, as certified by Food and Drugs Department with appropriate warehousing facilities, will automatically qualify and are eligible for 10% price advantage compared to imported items.
Need for Two-Stage Evaluation
It would appear that although a supplier may score the required points for prequalification, the company would still not be eligible if it does not meet any of the three criteria referred to in the previous paragraph. It is also not clear whether a supplier has to achieve the maximum points in relation to these criteria and how partial achievement would be dealt with. It would have been more appropriate to separate out these three items from the points system and apply a two-stage evaluation system. The first is to determine whether a potential supplier has satisfactorily demonstrated that his/her company has the financial and infrastructure capability as well as the ability to supply 75 per cent of the required items. If that determination is positive, the second stage is applied. This involves assessing the supplier on the other criteria set out in the above table. To have both sets of evaluation criteria together using a points system complicates the evaluation exercise.
Lack of Consultation
There was no evidence that manufacturers and distributors were consulted on the revised evaluation criteria. Had such consultations taken place, the Ministry would have been in a position to obtain valuable feedback, especially where the revised criteria are perceived to favour a particular supplier and/or place a particular supplier in a position of disadvantage. The new criteria therefore appear to be arbitrary and an imposition without the involvement of and consultation with those who are most affected – the suppliers. It is therefore not surprising that allegations of bias have surfaced even before the results of the prequalification exercise were announced. That apart, suppliers needed time to put in place arrangements to satisfy the new criteria, especially as regards the 30,000 square feet of storage with temperature-controlled environment.
Financial Capacity
While there is merit in ensuring that suppliers have the financial capacity to deliver on a contract, the requirements for a turnover over $1 billion, net assets over $500 million, at least $50 million per annum in corporate taxes and over 50 full-time employees appear too stringent. They would have placed small and medium sized suppliers in a position of disadvantage vis-à-vis the large suppliers. The normal practice is for contractors to lodge performance bonds with the procuring entity. If a contractor fails to deliver in a satisfactory manner, the procuring entity can levy against the bond. This is in addition to penalty clauses that are usually contained in contracts as well as the possibility of blacklisting.
Storage Facilities
The requirement for 30,000 square feet of storage with controlled temperature also appears too stringent, especially as regards distributors. They would normally import drugs and medical supplies based on purchase orders placed and do not need elaborate storage facilities compared with manufacturers. As soon as the items are received in the country, they could be delivered to the procuring entity, thereby obviate the need for elaborate storage facilities. Notwithstanding this, one of the applicants for prequalification claimed that the procuring entity did not carry out an inspection of the company’s warehousing facilities. However, according to an article from a newspaper that is affiliated to the company that was prequalified, the applicant was disqualified for failure “to provide the authorizations of the manufacturers of drugs”. Needless to mention, this is not one of the three mandatory requirements.
Another applicant, whose company is a distributor, began constructing warehouse in order to meet the infrastructure requirement. However, the evaluators suddenly turned up on 30 April 2014 to inspect the facility under construction, and according to the same newspaper, they found poor ventilation and “boxes thrown about and not properly packed”. The company was not given enough time to get its act together. What will happen to the investment made so far?
Experience and Proven Track Record
Applicants for prequalification must have more than seven years’ experience in supplying the Ministry without any adverse reports as well as a proven track record in handling contracts over $500 million with the Ministry. However, a review of the Auditor General’s reports up to 2012 would suggest that only the company that was prequalified would have met the requirement in terms of experience. This company has been the main supplier of pharmaceutical products over the years, accounting for over 75 per cent of the Government’s requirements.
In addition, the Auditor General’s reports over the years revealed a number of unsatisfactory features in respect of the performance of this company. For example, according to his 2012 report, medical supplies valued at $58.583 million were not delivered to the Georgetown Hospital as at 30 September 2013 and the related bank guarantee had expired in April 2013. A similar observation was made in respect of the Ministry of Health where medical supplies valued at $164.603 million had not been delivered, and there were no bank guarantees in force to cover this amount. There were also outstanding deliveries for 2011 totalling $59.835 million while for 2008 there was no evidence of the delivery of supplies valued at $79.262 million. Despite these shortcomings, no action was taken against the supplier for non-compliance with its contractual obligations.
To be continued –