(Part I)
During the last week or so, the purchase of drugs and medical supplies for the Ministry of Health and the Georgetown Hospital once again made headline news in the print media. The issue at hand was the release of a set of evaluation criteria to be used to prequalify suppliers for the purchase of these essential items. The media reports claimed that the criteria were heavily weighted in favour of a local organisation that has been the Government’s main supplier since 2005.
Procurement arrangements prior to 2004
Up to 2003, there was no legislation governing public procurement. The Tender Board Regulations, supplemented from time to time by Cabinet decisions, were used but they did not have the force of the Law. Successive reports of the Auditor General from 1992 onwards were, however, fraught with incidents of violation of these regulations, especially splitting of contracts to avoid adjudication by the higher authority levels and to favour particular suppliers and contractors. The Police, the Army and the Supreme Court were the main violators.
Cabinet’s decision of May 1997 authorized the Ministry of Health and the Georgetown Hospital to the purchase of drugs and medical supplies from specialized overseas agencies based on a system of selective tendering. This is different from prequalification proceedings in that these agencies were contacted and requested to tender for the supply of the required items. On the basis of their submissions, contracts were awarded. The main suppliers were the Pan American Health Organisation, United Nations Children’s Fund and the International Dispensary Association. Over 90 per cent of drugs and medical supplies were purchased from these agencies up to the end of 2004.

Prequalification requirements
The Procurement Act provides for the prequalification of suppliers or contractors. For certain types of goods/services and works, especially those of a repetitive nature, prequalification proceedings are advantageous in order to reduce the administrative burden in assessing large number of tenders each time invitations to bid are issued. Invitations to pre-qualify are to be published “in newspapers of wide circulation and posted in public places. Such solicitations shall reach the area impacted by the procurement”.
Among the prequalification documents to be issued to interested suppliers or contractors are:
● instructions for preparing and submitting prequalification applications;
● a summary of the required terms and conditions of the contract to be entered into
as a result of the procurement proceedings;
● any documentary evidence or other information that must be submitted by suppli
ers or contractors to demonstrate their qualifications; and
● the manner and place for the submission of applications to prequalify and the dead
line for such submission.
In reaching a decision regarding the qualifications of each supplier or contractor, the procuring entity is required to apply only the criteria set forth in the prequalification documents. Implicit in this, is the need for a document that highlights the evaluation criteria to be included in the package of prequalification documents so that prospective suppliers or contractors can ascertain the basis under which they will be evaluated.
The results of the prequalification proceedings must be communicated to all suppliers or contractors who have submitted applications to prequalify, and to any member of the public upon request. A supplier or contractor, who has applied for prequalification but does not satisfy the prequalification requirements, may request a review of the procuring entity’s decision. Only suppliers or contractors who have been prequalified are eligible to bid when invitations are solicited.
Analysis of the purchase of drugs and medical supplies:
Since 2004, a local organisation became the main supplier of drugs and medical supplies, virtually replacing the specialized overseas agencies that had previously supplied approximately 90 per cent of the Government’s requirement. During the period 2005 to 2012, the Government procured an estimated $20.5 billion worth of drugs and medical supplies. Of this amount, approximately $16 billion or 78 per cent was supplied by the local organization.
In 2005, the local organisation supplied $973.549 million in drugs and medical supplies. During the period 2007 to 2012, the value of purchases from this entity increased significantly to $3.033 billion, or threefold, by the end of 2012. I acknowledge that there would be price increases from one year to the next to cater for, among others, inflation and that the volume of purchases may change over time. Further analysis of this significant increase would therefore be needed to determine the amount that relates to pricing and whether such increases are reasonable. The Ministry did indicate that it has mechanisms in place to verify that price increases are reasonable. However, it is preferable for an independent assessment to be carried out.
Evaluation criteria for prequalification
I have seen a copy of a document dated October 2010 entitled “Prequalification Questionnaire” issued by the Ministry of Health for the procurement of drugs and medical supplies. It contains the following:
● Request for Prequalification (RFP) advertisement which emphasizes the need for suppliers to be legally registered in Guyana and Caribbean and to be principally involved in manufacturing and/or distribution of drugs;
● A statement that the NPTAB has approved of the prequalification of international suppliers – PAHO, UNICEF, UNFPA, UNDP and IDA, and that the invitation is an additional call; and
● A prequalification questionnaire that consists of four parts: business information, manufacturing information, quality information, and product information. All suppliers must be able to supply at least 75% of the number of line items on each list in order to be considered for prequalification.
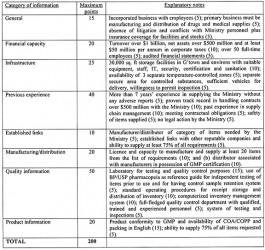
There was, however, no a document that highlighted the agreed evaluation criteria to be used so that prospective suppliers could ascertain how they would be evaluated. The evaluation criteria now being discussed in the print media, as part of the prequalification proceedings for the period 2014 to 2016, appear to be an attempt to ensure compliance with the Act. The document contains eight parts with a total maximum score of 200, as shown below:
Manufacturers are evaluated on a score of 190 while for distributors the score is 180. At least 80 per cent is required for prequalification in addition to meeting the criteria dealing with financial and infrastructure capacity as well as the ability to supply 75 per cent of GMA certified items. Preference is given to pharmaceutical manufacturers in Guyana as certified by Food and Drugs Department They automatically qualify and are eligible for 10% price advantage compared to imported items. Companies with appropriate warehousing facilities are also given preference.
Next week, we will examine further these evaluation criteria to ascertain whether there is merit in the claim that they were weighted in favour of any particular supplier.