The Government Analyst- Food and Drug Department (GA-FDD) has launched an investigation into the circulation of substandard/falsified (SF) medication on the local market and the police force’s fraud squad has been called in to assist with the probe.
This was confirmed yesterday by Director of the GA-FDD Marlon Cole, who told Stabroek News that the investigation was launched on August 22nd after the department received a complaint of an unknown individual either illegally manufacturing or importing the medication and releasing same for sale at retail pharmacies.
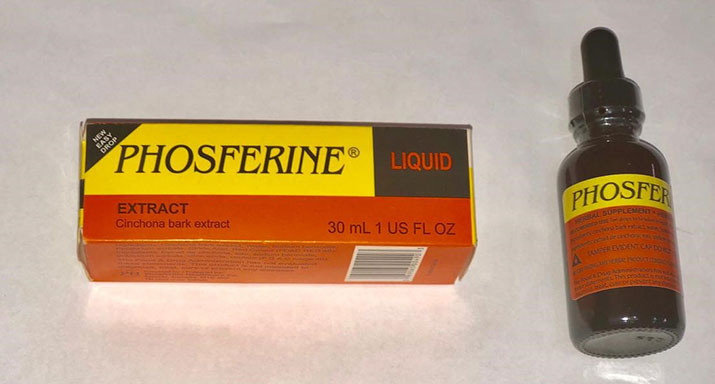
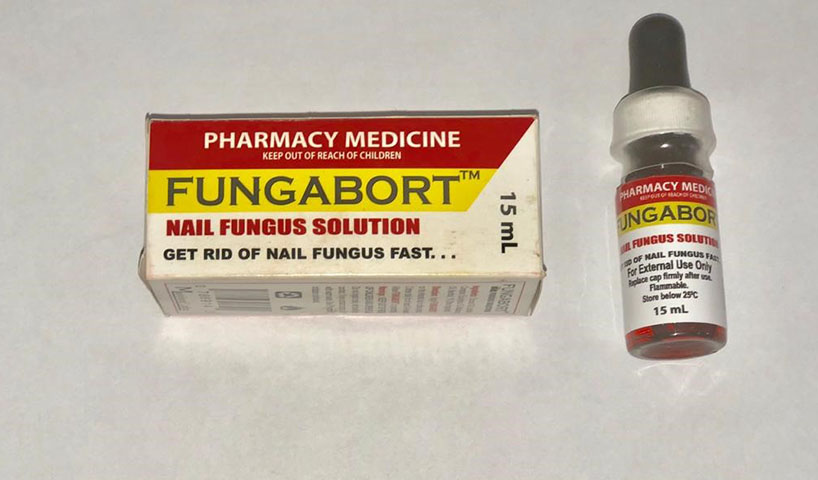
The medication that was seized and removed are Phosmovite, Fungabort and Phosferine, which were retailed on average at $3,500, $1,000 and $3,000, respectively.
In a press release issued last week, the GA-FDD said that during the initial stage of the investigation, its inspectors visited a total of 16 pharmacies in and around Georgetown within a two-day period during which a quantity of the medication was seized and removed.
At nine of the 16 pharmacies, inspectors unearthed a total of 17 boxes of a medication labelled “Chanca Piedra Phosmovite,” with a manufacturer’s address stated only as “Mainland Labs in Canada.” It claimed to treat gall and kidney stones, clean the liver and the urinary tract. Inspectors also found 28 boxes of “Fungabort,” with the same manufacturer’s address, which claimed to be effective in the treatment of nail fungus.”
In addition, 23 boxes of Phosferine were also seized and removed since the stated address “Phosferine Health Care Co., Toronto Canada” could not have been established by the department.
GA-FDD said efforts to verify the address on the labels of the other two products were also unsuccessful.
The release further stated that while it sought to locate the wholesaler/distributor, none of the pharmacies were able to provide receipts or other documentation as proof of purchase.
It called this “a clear breach” of the Consumer Affairs Act and the Food and Drug’s Act, which speak to adequate record keeping for traceability purposes, particularly for the sale of medication for patient use.
Cole said that the findings of the GA-FDD probe have been handed over to the Guyana Police Force’s fraud department, which is currently dealing with the matter.
He noted that they were working together to apprehend the perpetrator.
The GA-FDD statement said that the World Health Organization (WHO) reported in November, 2017 that SF medication distributed in low and middle income countries accounts for 10 % or 1 in 10 of all medication distributed, and cost these countries in excess of USD30 million annually.
The cost to the local economy and the percentage of SF medication is currently unknown.